Chromium trioxide
Agent Name
Chromium trioxide
Alternative Name
Chromium anhydride
CAS Number
1333-82-0
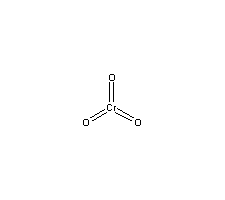
Formula
Cr-O3
Major Category
Metals
Synonyms
Chromium anhydride; Anhydride chromique [French]; Anidride cromica [Italian]; Chrome (trioxyde de) [French]; Chromia (CrO3); Chromic acid, solid; Chromic anhydride; Chromic oxide; Chromic(VI) acid; Chromium oxide; Chromium oxide (Cr4O12); Chromium oxide (CrO3); Chromium trioxide; Chromium(6+) oxide; Chromium(6+) trioxide; Chromium(VI) oxide; Chromsaeureanhydrid [German]; Chromtrioxid [German]; Chroomtrioxyde [Dutch]; Chroomzuuranhydride [Dutch]; Cromo(triossido di) [Italian]; Monochromium trioxide; Puratronic chromium trioxide; Sintered chromium trioxide; [ChemIDplus] UN1463
Category
Chromium Compounds, Inorganic
Description
Dark red to brown crystals, flakes, or powder; [HSDB] Soluble in water; [ACGIH]
Sources/Uses
Used in chromium plating, copper stripping, and aluminum anodizing; also used to inhibit corrosion, to develop photographs, to purify oil and acetylene, to harden microscopic preparations, and to oxidize organic chemicals; [HSDB] Used in sulfuric acid as a photoresist stripper; [CSH, p. 49] Also used as a wood preservative; [ACGIH]
Comments
CrVI compounds are irritants, sensitizers, and may be corrosive to the skin. CrVI compounds are respiratory tract irritants and may cause pulmonary sensitization. CrVI may cause acute tubular necrosis and liver injury. [ATSDR Case Studies] Highly corrosive to skin; [Quick CPC] Chromium trioxide (chromic acid) is a water-soluble hexavalent chromium compounds. A corrosive substance that can cause injury to the skin, eyes, and respiratory tract; May cause skin sensitization, kidney injury, and asthma after prolonged contact; [ICSC] See "Chromium" and linked occupational diseases.
Biomedical References
Exposure Assessment
Skin Designation (ACGIH)
Yes
Bioaccumulates
Yes
TLV (ACGIH)
0.0002 mg/m3, as Cr(VI), inhalable particulate matter
STEL (ACGIH)
0.0005 mg/m3, as Cr(VI), inhalable particulate matter
PEL (OSHA)
0.005 mg/m3, as Cr(VI)
Lethal Concentration
LC50 (rat) = 167 mg/m3/4 H; [CHEMINFO]
Explanatory Notes
Melting point = 197 degrees C; [HSDB] The Guide from the Emergency Response Guidebook is for "Chromium trioxide, anhydrous."
NFPA
will not burn
Adverse Effects
Skin Sensitizer
Yes
Asthma
Yes
Hepatotoxin
Hepatoxic (a) from occupational exposure (secondary effect) or (b) in animal studies or in humans after ingestion
Nephrotoxin
Yes
Dermatotoxin
Skin burns
IARC Carcinogen
Established
NTP Carcinogen
Human carcinogen
ACGIH Carcinogen
Confirmed Human
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent:
Processes
Industrial Processes with risk of exposure:
Activities
Activities with risk of exposure: