Formothion
Agent Name
Formothion
CAS Number
2540-82-1
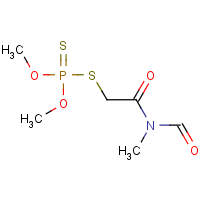
Formula
C6-H12-N-O4-P-S2
Major Category
Pesticides
Synonyms
2-Dimethoxyphosphinothioylthio-N-formyl-N-methylacetamide; Acetamide, N-formyl-2-mercapto-N-methyl-, S-ester with O,O-dimethyl phosphorodithioate; Aflix; Afliz; Afliz [Sandoz]; Anthio; Anthio 25; Antio; CP 53926; ENT 27,257; Formothion 25 EC; J-38; N-Formyl-N-methylcarbamoylmethyl O,O-dimethyl phosphorodithioate; O,O-Dimethyl S-(N-formyl-N-methylcarbamoylmethyl) phosphorodithioate; O,O-Dimethyl S-(N-methyl-N-formylcarbamoylmethyl)phosphorodithioate; O,O-Dimethyl dithiophosphorylacetic acid N-methyl-N-formylamide; O,O-Dimethyl phosphorodithioate N-formyl-2-mercapto-N-methylacetamide S-ester; O,O-Dimethyl phosphorodithioate S-ester with N-formyl-2-mercapto-N-methylacetamide; O,O-Dimethyl-S-(3-methyl-2,4-dioxo-3-aza-butyl)-dithiofosfaat [Dutch]; O,O-Dimethyl-S-(3-methyl-2,4-dioxo-3-aza-butyl)-dithiophosphat [German]; O,O-Dimethyl-S-(N-methyl-N-formyl-carbamoylmethyl)-dithiophosphat [German]; O,O-Dimetil-S-(N-formil-N-metil-carbamoil-metil)-ditiofosfato [Italian]; OMS-968; P 1; Phosphorodithioic acid, O,O-dimethyl ester, N-formyl-2-mercapto-N-methylacetamide S-ester; Phosphorodithioic acid, S-(2-(formylmethylamino)-2-oxoethyl) O,O-dimethyl ester; S 6900; S-(2-(Formylmethylamino)-2-oxoethyl) O,O-dimethyl phosphorodithioate; S-(2-(Formylmethylamino)-2-oxoethyl) O,O-dimethylphosphorodithioate; S-(Formyl(methyl)carbamoylmethyl) O,O-dimethyl phosphorodithioate; S-(N-Formyl-N-methylcarbamoylmethyl) O,O-dimethyl phosphorodithioate; S-(N-Formyl-N-methylcarbamoylmethyl) dimethyl phosphorothiolothionate; SAN 244 I; SAN 6913 I; SAN 7107 I; Sandoz S-6900; Spencer S-6900; Toprose; VEL 4284; [ChemIDplus] UN3018
Category
Organophosphate Insecticides
Description
Yellow liquid; mp = 25 deg C; [Hawley] Pale yellow liquid or solid; mp = 25-26 deg C; [HSDB] Colorless to pale yellow liquid or solid; mp = 25-26 deg C; [MSDSonline]
Sources/Uses
Used as acaricide and systemic insecticide; [Merck Index] Not currently commercially produced in the US; Likely discontinued for pesticide use worldwide; [HSDB]
Comments
Not irritating to rabbit eyes; Effects in high dose animal studies include inhibition of cholinesterase activity, minor histological changes to liver and adrenal glands, functional changes of the liver, and hematological changes; No evidence of skin sensitization, increased tumor incidence, other neurotoxicity, nor developmental or teratogenic toxicity [HSDB] No significant adverse gross or histological changes or increase in tumor frequency observed in two year feeding studies with rats and dogs; [INCHEM JMPR] “The average of two baseline respective cholinesterase activity determinations three days apart, with no exposures to enzyme inhibiting pesticides for at least 30 days, is recommended for each worker prior to exposure to cholinesterase inhibitors because of large inter-individual differences in published baseline values. To be established at least once a year. Removal from workplace exposures is recommended until the cholinesterase activity returns to within 20% of baseline.” [TLVs and BEIs]
Biomedical References
Exposure Assessment
BEI
Acetylcholinesterase activity in red blood cells = 70% of individual's baseline; Butylcholinesterase activity in serum or plasma = 60% of individual's baseline; Sample at end of shift; [TLVs and BEIs]
Vapor Pressure
8.48E-07 mm Hg
Lethal Concentration
LC50 (rat) = 4,500 mg/m3/4h
Explanatory Notes
The Guide in the Emergency Response Guidebook is for "Organophosphorus pesticide, liquid, toxic."
Adverse Effects
Other Poison
Organophosphate
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent:
Processes
Industrial Processes with risk of exposure: