Zinc chloride fume
Agent Name
Zinc chloride fume
CAS Number
7646-85-7
Formula
Cl2-Zn
Major Category
Metals
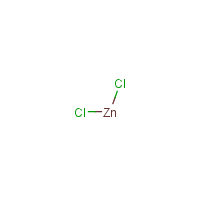
Synonyms
Butter of zinc; Chlorure de zinc [French]; Zinc (chlorure de) [French]; Zinc butter; Zinc chloride; Zinc chloride in plastic container; Zinc chloride, (solution); Zinc dichloride; Zinc(II) chloride; Zinco (cloruro di) [Italian]; Zine dichloride; Zinkchlorid [German]; Zinkchloride [Dutch]; Zintrace; Zinc chloride, anhydrous; Zinc chloride, solution; [ChemIDplus] UN2331; UN1840 (solution)
Category
Metals, Inorganic Compounds
Description
White particulate dispersed in air; [NIOSH] Zinc chloride: White odorless solid; Highly deliquescent; [Merck Index] White odorless powder, crystals, or beads; Hygroscopic; [Alfa Aesar MSDS]
Sources/Uses
Zinc chloride is used as a soldering flux, battery electrolyte, and crowd control agent; also used in iron galvanizing, textiles, adhesives, deodorants, wood preserving, and petroleum refining; [ACGIH] Used as a glazing flux in glassblowing; [www.ci.tucson.az.us/arthazards/medium.html] Other applications include deodorizing, disinfecting, embalming, fireproofing lumber, etching metals, microscopy, chemical syntheses, making parchment paper, artificial silk, dyes, activated carbon, cold-water glues, and vulcanized fiber, browning steel, copper-plating iron, magnesia cements, textiles (mordant in printing and dyeing, carbonizing woolen goods, mercerizing cotton, and sizing and weighting fabrics), vulcanizing rubber, cellulose solvent, dentin desensitizer, smoke bombs, and as human and veterinary astringent [Merck Index] Registered for use in the US for the control of moss and fungi on outdoor walkways and patios; [EPA REDs]
Comments
Asthma reported in a solderer and a locksmith; [Malo] At a concentration of 0.4 mg/m3, ZnCl2 caused no respiratory irritation; at 4.8 mg/m3 for 30 minutes, transient respiratory irritation occurred. Higher concentrations after industrial accidents have been associated with toxic pneumonitis. [ACGIH] Zinc chloride is corrosive to the eyes, skin, and respiratory tract; [ICSC] Causes burns; Harmful by ingestion; [Alfa Aesar MSDS] See "Zinc."
Reference Link #1
Biomedical References
Exposure Assessment
Skin Designation (ACGIH)
Insufficient data
TLV (ACGIH)
1 mg/m3
STEL (ACGIH)
2 mg/m3
PEL (OSHA)
1 mg/m3
MAK
0.1 mg/m3 (respirable fraction), 2 mg/m3 (inhalable fraction)
IDLH (NIOSH)
50 mg/m3
Excerpts from Documentation for IDLHs
Human data: A 30minute exposure to 4.8 mg/m3 has been reported to produce respiratory distress [Ferry 1974]. Exposure to 80 mg/m3 has caused nausea and coughing and 120 mg/m3 for 2 minutes has caused nose and upper respiratory system irritation [Cullumbine 1957].
Lethal Concentration
LCLo (rat) = 1,960 mg/m3/10min
Explanatory Notes
LC50 (rat) = 2,000 mg/m3
Half Life
For zinc, whole body: 162-500 days; [TDR, p. 1245]
Reference Link #2
NFPA
will not burn
Adverse Effects
Asthma
Yes
Toxic Pneumonitis
Yes
Dermatotoxin
Skin burns
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent:
Processes
Industrial Processes with risk of exposure:
Activities
Activities with risk of exposure: