1. What is ionizing radiation?
Radioactive atoms decay spontaneously to emit radiation. The energy of the emitted radiation is sufficient to strip away electrons from other atoms, creating charged ions. Ionizing radiation can injure any tissue in the human body. The body can repair the damage if it is not too severe or widespread. Radioactive materials are chemical and radiation hazards. The degree of the radiation hazard varies with different materials and depends on the strength of the ionizing radiation, the half-life, and the amount. Radiation can cause injury after inhalation, ingestion, or direct (external) exposure. [EPA website: Understanding Radiation] Elements with the same number of protons and different number of neutrons are called isotopes. Isotopes have identical chemical properties, but some isotopes are stable and some are unstable (radioactive). The activity of a radionuclide (radioactive isotope) is a measure of how many atoms undergo radioactive decay per unit of time, i.e., disintegrations/sec. [WHO website: Ionizing radiation]
For tables of radionuclides (pdf files) giving information on half-lives, decay modes, radiation energies, and other facts, see Argonne National Laboratory: Summary Radioactive Properties for Selected Radionuclides and NCRP Report No. 65: Information on Selected Radionuclides, p. 14-17. This Table of Radionuclides from Vanderbilt University includes "Internal Toxicity Class."
2. How is it measured? Absorbed Dose
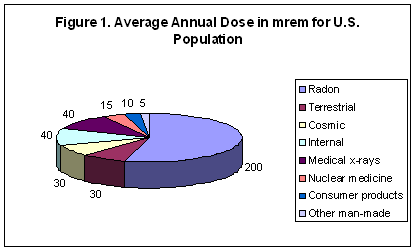
A millirem or mrem is 1/1000 of a rem, a measure of the biological effect of an absorbed dose of ionizing radiation. The average annual background dose for Americans is 360 mrem (0.36 rem). As shown in Figure 1, 55% of the average background dose comes from radon. Also contributing to natural background doses are terrestrial (8%), cosmic (8%), and internal (11%) sources. A chest x-ray delivers about 10 mrem. A bone scan delivers about 630 mrem. A passenger receives about 5 mrem on a transcontinental flight across the United States. Each individual receives about 20 mrem per year from radioactive potassium (K-40) and 20 mrem from other internal radionuclides for a 40 mrem per year total internal radiation dose. [Argonne Radiological Fact Sheets]
Units in the metric system are the Gray (Gy) and the Sievert (Sv).
Dose absorbed (energy deposited): 100 rad = 1 Gray;
Dose absorbed (biological effect): 100 rem = 1 Sievert; Also called the dose equivalent;
100 mrem = 1 mSv;
1 rem = 1 rad for x-rays and gamma rays;
3. How is it measured? Activity of Radioactive Materials
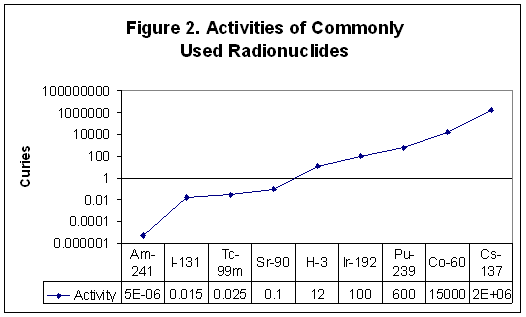
While the rem measures the dose absorbed by tissues, the curie measures the activity or intensity of the source of radioactivity. One curie is the amount of activity in one gram of radium-226. In one curie of material, 3.7 x 1010 atoms disintegrate (emit radiation) every second. The radionuclides listed in Figure 2 are products of nuclear reactors or accelerators. The figure shows the amount in curies used for Americium-241 in smoke detectors, Iodine-131 in nuclear medicine therapy, Technetium-99m in diagnostic imaging, Strontium-90 in eye therapy, Tritium (H-3) in exit signs, Iridium-192 in industrial radiography, Plutonium-239 in nuclear weapons, Cobalt-60 in cancer therapy, and Cesium-137 in food irradiators. [From a PowerPoint presentation by the Health Physics Society]
Radium, radon and potassium-40 (K-40) are natural sources of radiation and three of the hundreds of radioactive isotopes (also called radionuclides). "Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons." The human body contains about 0.1 microcuries (0.000001 curies) of K-40. The main naturally occurring isotopes of potassium are K-39, K-41, and K-40. The first two isotopes are stable and nonradioactive. K-39 comprises about 93% of naturally occurring potassium, K-41 about 7%, and K-40 about 0.012%. Argonne Radiological Fact Sheets
37 GBq (gigabecquerel) = 1 Ci (curie);
37 MBq (megabecquerel) = 1 mCi (millicurie); 1 MBq = 27 uCi;
37 kBq (kilobecquerel) = 1 uCi (microcurie)
37 Bq (becquerel) = 1 nCi (nanocurie) = 37 disintegrations per second (dps)
4. What are the limits for acute exposure? Dose Rates
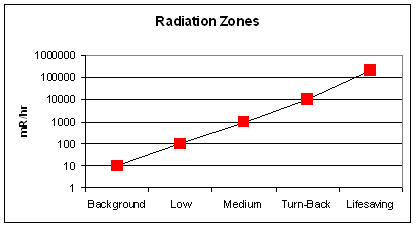
- Turn-back zone (1,000-10,000 mR/hr): time limit is 30 min. to 5 hr;
- Lifesaving zone (>10,000 mR/hr): time limit is min. to a few hr;
- Emergency workers in an area with 200,000 mR/hr would receive a dose of 50,000 mrem in just 15 min. They would be at risk for acute radiation syndrome after receiving doses of 200,000 mrem or greater.
Assume 1 milliroentgen (mR) = 1 millirem (mrem). Conference of Radiation Control Program Directors: First Responder's Guide
Typical background levels are 50 counts per minute (cpm) with a surface contamination meter and 0.02 mrem/hr with a gamma dose rate meter. The Canadian Nuclear Safety Commission: Working in a Radiation Environment
Cosmic ray dose at 30,000 to 40,000 feet = 1 mrem/hr.
Dose from monazite sand containing natural radioactive thorium in Brazil = 5 mrem/hr. ATSDR Toxicological Profile: Ionizing Radiation, p. 234.
Orange dinnerware and pottery glazed with uranium, a common practice up until a few years ago, shows a reading of tens of mR/hr on a geiger counter. [Gollnick, p. 37]
"Levels in the cpm range and millirad range are associated with a low-level risk to the medical personnel. Only in the rad/hr (Sv/hr) range would it be necessary to institute more stringent radiation protective procedures in non-life saving situations." Disaster Preparedness for Radiology Professionals
5. What are the limits for chronic exposure? Accumulated Dose
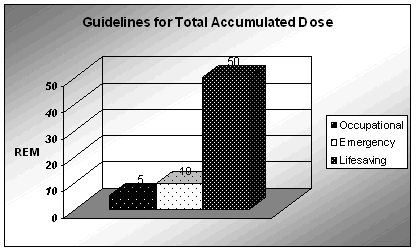
Five rem (5000 mrem) is the standard occupational dose limit for one year. The other two accumulated dose limits are for non-lifesaving activities involving critical property protection (10 rem) and lifesaving activities (50 rem). Conference of Radiation Control Program Directors: First Responder's Guide
"Conclusive evidence that ionizing radiation causes cancer comes from the studies of radium dial painters, underground miners, pioneering radiologists, patient populations, and Japanese atomic bomb survivors." For radium dial painters, no bone cancer was observed when doses were less than 1000 rads to the bone. For underground miners, no increased risk was found for miners receiving less than 250 rem. Pioneering radiologists who had increased incidence of leukemia and skin cancer received an estimated 100 to 800 rads or more. Workers at the Mayak nuclear complex in Russia had increased incidence of lung, liver, and bone cancer after inhaling very large doses of plutonium during the period of 1948-1958. [Boice, p. 259-276] Plutonium workers in the U.S. were followed for decades and no increase in cancer was found. A ten year study of nuclear shipyard workers included 28,582 workers with doses over 500 mrem. This exposed group had lower than expected cancer rates except for mesothelioma attributable to asbestos exposure. 28,855 Japanese survivors exposed to doses in the range of 500 mrem to 5000 mrem, had 108 fewer cancer deaths than expected compared to controls. [Gollnick, p. 110-112] "To date, the most reliable studies of the effects of radiation exposure at low levels received by occupational workers have not been able to detect adverse effects associated with their radiation exposure except at the higher doses, i.e., greater than approximately 10 rem." [Position Statement of the Health Physics Society]
6. What are the main principles of radiation protection?
| Minimize Time | Dose = dose rate x time. |
| Maximize Distance | Dose is 4 times less at double the distance from the source and 9 times less at triple the distance. |
| Incorporate Shielding | Alpha stopped by a sheet of paper; Beta by a sheet of plastic or glass; Gamma by lead; |
The Canadian Nuclear Safety Commission: The Basics of Ionizing Radiation
7. For further information, see "Radiological Terrorism for Healthcare" viewed as one of the Medfilms Online Courses at UAHS Health Sciences. Facts from that film:
- Only one half of one percent of the 85,000 Japanese survivors of the atomic bombs developed cancer attributed to this radiation exposure.
- The maximum dose received by health care workers treating the victims of the Chernobyl accident was 1 rad. (One rad is the dose received from a CT of the abdomen.)
Revised: May 30, 2018