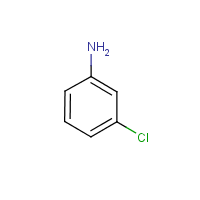
3-Chloroaniline
Agent Name
3-Chloroaniline
Alternative Name
m-Chloroaniline
CAS Number
108-42-9
Formula
C6-H6-Cl-N
Major Category
Nitrogen Compounds
Synonyms
m-Chloroaniline; 1-Amino-3-chlorobenzene; 3-Chlooranilinen [Dutch]; 3-Chloroaniline; 3-Chlorobenzenamine; 3-Chlorophenylamine; 3-Cloroaniline [Italian]; AI3-12126; Aniline, m-chloro-; Benzenamine, 3-chloro-; Fast Orange GC Base; Orange GC Base; m-Aminochlorobenzene; m-Chloraniline; m-Chloroaminobenzene; m-Chloroaniline; m-Chlorophenylamine; meta-Aminochlorobenzene; [ChemIDplus] UN2019
Category
Amines, Aromatic
Description
Colorless to light amber, tends to darken during storage; [HSDB]
Sources/Uses
Used as an intermediate to produce herbicides, pharmaceuticals, azo dyes, and pigments; [HSDB]
Comments
"In the United Kingdom between 1961 and 1980, chloroaniline, p-toluidine, nitrobenzene, and nitrochlorobenzene were the most common industrial causes of methemoglobinemia. Dermal exposure was a more frequent route of toxicity than inhalation with these compounds." (Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988.) A skin and eye irritant; Can induce methemoglobinemia; [ICSC] Danger of skin sensitization; [MAK]
Biomedical References
Exposure Assessment
BEI
Methemoglobin in blood = 1.5% of hemoglobin during or at end of shift. [ACGIH]
Vapor Pressure
0.066 mm Hg
Lethal Concentration
LC50 (mice) = 550 mg/m3/4h
Explanatory Notes
Flash point = 255 deg F; VP from HSDB;
Reference Link #2
Adverse Effects
Skin Sensitizer
Yes
Methemoglobinemia
MetHgb is primary toxic effect
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent: