Bromine trifluoride
Agent Name
Bromine trifluoride
CAS Number
7787-71-5
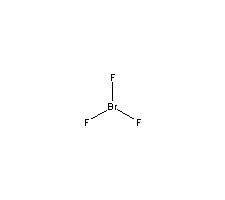
Formula
Br-F3
Major Category
Toxic Gases & Vapors
Synonyms
Bromine fluoride; [ChemIDplus] UN1746
Category
Oxidizers
Description
A colorless to yellow, fuming liquid with a pungent odor; mp = 8.77 deg C; [CAMEO] Yellow or gray liquid with an extremely irritating odor; [Airgas MSDS]
Sources/Uses
Used as a solvent and fluorinating agent; Used in uranium enrichment and fuel element reprocessing; [HSDB]
Comments
Highly corrosive to skin; [Quick CPC] 100 ppm is a lethal concentration for some laboratory animals. High inhalation exposure can induce pulmonary edema. [HSDB] Reacts explosively with water evolving oxygen; Can cause severe burns to skin and eyes; A severe respiratory tract irritant; [CAMEO] Highly reactive and smokes in air; Corrosive and irritating; [Merck Index] Reacts violently with water producing hydrogen fluoride gas; Causes severe burns to skin and eyes; Inhalation causes severe upper respiratory tract irritation; [CHRIS] A strong oxidizing agent that can cause fire on contact with combustible materials; Reacts with water evolving flammable and toxic gases; Corrosive to skin, eye, and respiratory tract; May be fatal by inhalation; [Airgas MSDS]
Biomedical References
Exposure Assessment
TIH
Yes
Dangerous When Wet
Yes
Explanatory Notes
VP =18 mm Hg at 39 degrees C; [HSDB] Some “Water Reactive Materials” are also TIH materials themselves: Bromine trifluoride; [ERG 2016]
NFPA
will not burn
Adverse Effects
Toxic Pneumonitis
Yes
Dermatotoxin
Skin burns
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent:
Processes
Industrial Processes with risk of exposure: