Sulfuryl chloride
Agent Name
Sulfuryl chloride
CAS Number
7791-25-5
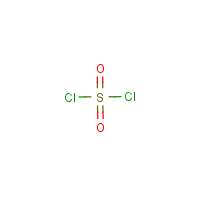
Formula
Cl2-O2-S
Major Category
Toxic Gases & Vapors
Synonyms
Sulfonyl chloride; Sulfonyl dichloride; Sulfur oxychloride (SO2Cl2); Sulfuric dichloride; Sulfuric oxychloride; Sulfuryl dichloride; Sulphuryl chloride; Sulphuryl dichloride; [ChemIDplus] UN1834
Category
Acid Halides
Description
Colorless to yellow liquid with a pungent odor; [ICSC]
Sources/Uses
Used as a chemical intermediate and to make shrink-resistant wool; [HSDB] A basic chemical used to make intermediates for various industries and in hermetically sealed high power batteries with specialized applications; [Reference #1]
Comments
Corrosive to skin; [Quick CPC] High inhalation exposure may induce pneumonitis and pulmonary edema; [ICSC] Sulfuryl chloride release HCl when spilled in water; [ERG 2016] "Even the vapors are corrosive to human skin and mucous membranes." Slowly decomposed by water producing sulfuric and hydrochloric acids; [Merck Index] Reacts violently with water and is slowly hydrolyzed by moist air producing chlorosulfonic, sulfuric, and hydrochloric acids; Reports of delayed onset pulmonary edema in humans following inhalation exposure; [Reference #1] A lachrymator; [CHEMINFO]
Reference Link #1
Biomedical References
Exposure Assessment
TIH
Yes
Dangerous When Wet
Yes
Vapor Pressure
140 mm Hg
Lethal Concentration
LC50 (rat) = 159 ppm/4hr
Explanatory Notes
LC50 (rat) = 878 mg/m3/4hr; [Reference #1] Some “Water Reactive Materials” are also TIH materials themselves: Sulfuryl chloride; [ERG 2016] VP from HSDB;
Reference Link #2
NFPA
will not burn
ERPG-1
0.3 ppm
ERPG-2
3 ppm
ERPG-3
15 ppm
Adverse Effects
Lachrymator
Yes
Toxic Pneumonitis
Yes
Dermatotoxin
Skin burns
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent:
Processes
Industrial Processes with risk of exposure: