Calcium carbide
Agent Name
Calcium carbide
CAS Number
75-20-7
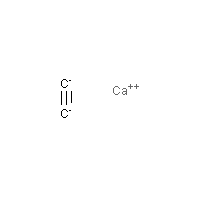
Formula
C2-Ca
Major Category
Metals
Synonyms
Acetylenogen; Calcium acetylide (Ca(C2)); CaC2; Calcium dicarbide; Carbure de calcium [French]; Carburo calcico [Spanish]; Ethyne, calcium deriv.; UN1402; [ChemIDplus]
Category
Metals, Inorganic Compounds
Description
Grayish-black, irregular lumps or crystals with a garlic odor; [CHEMINFO]
Sources/Uses
Used to desulfurize steel and iron, to produce acetylene, to reduce copper sulfide to metallic copper, and to weld and cut metals; [HSDB]
Comments
Explosive hazard; Corrosive to the skin, eyes, and respiratory tract; Inhalation can cause pulmonary edema; [ICSC] If left on clothes, may cause reddening of skin; [CHRIS] Forms flammable gas (acetylene) on exposure to moisture or water; [CHEMINFO] Carbides are one of the four binary salts that have specific hazards (nitrides, carbides, hydrides, and phosphides); Carbides give off acetylene, and a corrosive base is formed from contact with water. The corrosive base is the hydroxide of the metal that is attached to the carbon in the carbide compounds. On contact with water, calcium carbide produces acetylene and the corrosive calcium hydroxide. [Burke, p. 21]
Biomedical References
Exposure Assessment
NFPA
may ignite at ambient temp
Adverse Effects
Toxic Pneumonitis
Yes
Dermatotoxin
Skin burns
Diseases, Processes, and Activities Linked to This Agent
Processes
Industrial Processes with risk of exposure: