Silver difluoride
Agent Name
Silver difluoride
CAS Number
7783-95-1
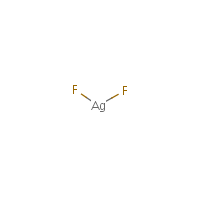
Formula
Ag-F2
Major Category
Metals
Synonyms
Argent fluorure [French]; Argentic fluoride; Silver (II) fluoride; Silver fluoride (AgF2); Silver(II) fluoride; [ChemIDplus] UN3084
Category
Metals, Inorganic Compounds
Description
White solid when pure; Highly hygroscopic; "Greasy black mass" when exposed to atmospheric moisture; [Merck Index] Brown crystalline solid; [Alfa Aesar MSDS]
Sources/Uses
Used in the fluorination of hydrocarbons; [Merck Index]
Comments
Reacts violently with water; A strong oxidizing agent; Highly toxic with symptoms due to fluoride; [Merck Index] Reacts violently with water; An oxidizer that may cause fire on contact with combustible materials; Causes burns; Inhalation may cause corrosive injuries to upper respiratory tract and lungs; Harmful by ingestion, inhalation, and skin absorption; [Alfa Aesar MSDS]
Biomedical References
Exposure Assessment
BEI
Fluorides in urine = 2 mg/L prior to shift or 3 mg/L at end of shift; (Repeated measurements recommended.)
TLV (ACGIH)
2.5 mg/m3, as F
PEL (OSHA)
2.5 mg/m3, as F
MAK
0.01 mg/m3, inhalable fraction, as Ag (salts) [1 mg/m3, as F, inhalable fraction]
Explanatory Notes
The Guide in the Emergency Response Guidebook is for "Corrosive solid, oxidizing, n.o.s."
Adverse Effects
Toxic Pneumonitis
Yes
Dermatotoxin
Skin burns
Diseases, Processes, and Activities Linked to This Agent
Other Information
No other related information on this agent was found.