Calcium fluoride
Agent Name
Calcium fluoride
CAS Number
7789-75-5
Formula
Ca-F2
Major Category
Other Classes
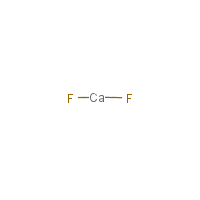
Synonyms
Acid-spar; Calcium difluoride; Calcium fluoratum; Calcium fluoride; Calcium fluoride (CaF2); Fluorite; Fluorspar; Fluorure de calcium; Irtran 3; Liparite; Met-spar; Natural fluorite; [ChemIDplus]
Category
Fluorides, Inorganic
Description
Gray powder that sinks in water; [CAMEO] White to clear crystals; Natural fluorite varies in color from clear to white, yellow, green, blue, purple, and brown; Some commercial grades of fluorite contain crystalline silica; [CHEMINFO]
Sources/Uses
The primary source of fluorine and fluorine compounds, it is used in the steel, chemical, ceramics, and glass industries. Used as a flux and catalyst; [Merck Index] Used as a welding flux; [Ullmann]
Comments
"Little acute toxicity;" [CAMEO] Normally stable, but decomposes into hydrogen fluoride when heated to 1200 deg C or mixed with concentrated acids; [CHEMINFO] See "FLUORIDES."
Biomedical References
Exposure Assessment
BEI
Fluorides in urine = 2 mg/L prior to shift or 3 mg/L at end of shift; (Repeated measurements recommended.)
TLV (ACGIH)
2.5 mg/m3, as F
PEL (OSHA)
2.5 mg/m3, as F
MAK
1 mg/m3, as F, inhalable fraction
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent:
Processes
Industrial Processes with risk of exposure: