Aspartame
Agent Name
Aspartame
CAS Number
22839-47-0
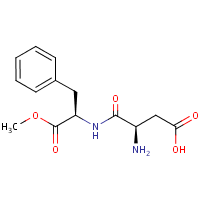
Formula
C14-H18-N2-O5
Major Category
Other Uses
Synonyms
1-Methyl N-L-alpha-aspartyl-L-phenylalanate; 1-Methyl N-L-alpha-aspartyl-L-phenylalanine; 3-Amino-N-(alpha-carboxyphenethyl)succinamic acid N-methyl ester; 3-Amino-N-(alpha-carboxyphenethyl)succinamic acid N-methyl ester, stereoisomer; 3-Amino-N-(alpha-methoxycarbonylphenethyl) succinamic acid; APM; Asp-phe-ome; Aspartam [INN-French]; Aspartame, L,L-alpha-; Aspartamo [INN-Spanish]; Aspartamum [INN-Latin]; Aspartylphenylalanine methyl ester; Canderel; Dipeptide sweetener; Equal; L-Aspartyl-L-phenylalanine methyl ester; L-Phenylalanine, N-L-alpha-aspartyl-, 1-methyl ester; Methyl aspartylphenylalanate; Methyl L-alpha-aspartyl-L-phenylalanate; Methyl L-aspartyl-L-phenylalanine; Methyl N-L-alpha-aspartyl-L-phenylalaninate; N-L-alpha-Aspartyl-L-phenylalanine 1-methyl ester; Nutrasweet; SC 18862; SC-18862; Succinamic acid, 3-amino-N-(alpha-carboxyphenethyl)-, N-methyl ester, stereoisomer; Sweet dipeptide; Tri-sweet; [ChemIDplus]
Category
Food Additives
Description
Colorless or white odorless solid; [HSDB] White powder; [Alfa Aesar MSDS]
Sources/Uses
Used as a non-nutritive sweetener; [Merck Index] Used as a flavor enhancer and non-nutritive sweetener; [FDA]
Comments
Causes effects on fluid intake, degenerative changes to brain, and effects on phosphatases and true cholinesterase in acute oral studies of rats; [RTECS] An eye and mucous membrane irritant; [IUCLID] Acceptable daily intake (ADI) of 0-40 mg/kg; [JECFA] May cause irritation; [Alfa Aesar MSDS]
Biomedical References
Exposure Assessment
Vapor Pressure
4.54E-12 mm Hg
Adverse Effects
IARC Carcinogen
Possible (2b)
Diseases, Processes, and Activities Linked to This Agent
Other Information
No other related information on this agent was found.