Selenium dioxide
Agent Name
Selenium dioxide
CAS Number
7446-08-4
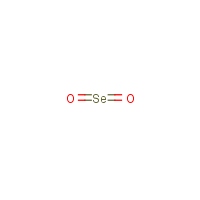
Formula
O2-Se
Major Category
Other Classes
Synonyms
Selenious anhydride; Selenium dioxide; Selenium dioxide dimer; Selenium oxide; Selenium oxide (1:2); Selenium oxide (Se2O4); Selenium oxide (SeO2); Selenium(IV) dioxide (1:2); Selenous acid anhydride; [ChemIDplus]
Category
Other Inorganic Compounds
Description
White or yellowish-white to slightly reddish, lustrous, crystalline powder or needles. [HSDB]
Sources/Uses
Used to make other selenium compounds; Also used as a reagent and oxidizing agent; [Merck Index #8434] Used as a red colorant in glass and a toner in photographic development; [Wikipedia]
Comments
Reacts with water to produce selenious acid; Can cause severe burns and irritation of the eyes and respiratory tract; A strong vesicant and is absorbed through the skin; [Olson, p. 761] A corrosive substance that can induce pulmonary edema; Has potential to cause skin sensitization and liver injury; [ICSC] See "Selenium."
Biomedical References
Exposure Assessment
TLV (ACGIH)
0.2 mg/m3, as Se
PEL (OSHA)
0.2 mg/m3, as Se
MAK
0.02 mg/m3, as Se, inhalable fraction
IDLH (NIOSH)
1 mg/m3, as Se
Explanatory Notes
VP 12.5 mm Hg @ 70 deg C; mp = 340 deg C; [Merck Index #8434]
Adverse Effects
Skin Sensitizer
Yes
Toxic Pneumonitis
Yes
Neurotoxin
Other CNS neurotoxin
Hepatotoxin
Hepatoxic (a) from occupational exposure (secondary effect) or (b) in animal studies or in humans after ingestion
Reproductive Toxin
Yes
Dermatotoxin
Skin burns
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent:
Processes
Industrial Processes with risk of exposure:
Activities
Activities with risk of exposure: