Chlorine trifluoride
Agent Name
Chlorine trifluoride
CAS Number
7790-91-2
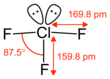
Formula
Cl-F3
Major Category
Toxic Gases & Vapors
Synonyms
Chlorine fluoride (ClF3); Chlorine trifluoride (ClF3); Chlorotrifluoride; Trifluorure de chlore [French]; [ChemIDplus] UN1749
Category
Oxidizers
Description
Colorless gas or a greenish-yellow liquid (below 53 degrees F) with a somewhat sweet, suffocating odor; Note: Shipped as a liquefied compressed gas; [NIOSH]
Sources/Uses
Used as a fluorinating agent and fire retardant for fluorocarbon polymers; also used to ignite rocket fuels and in the processing of fuels for nuclear reactors; [ACGIH]
Comments
Liquid causes second or third degree burns after short contact; [CHRIS] Chlorine trifluoride causes severe lung irritation and death in animal inhalation studies; [ACGIH] A corrosive substance that can cause pulmonary edema; [ICSC] See "FLUORIDES."
Biomedical References
Exposure Assessment
Skin Designation (ACGIH)
Insufficient data
TIH
Yes
Ceiling (ACGIH)
0.1 ppm
PEL (OSHA)
Ceiling(OSHA) = 0.1 ppm
IDLH (NIOSH)
20 ppm
Excerpts from Documentation for IDLHs
Other animal data: No mortality occurred among 2 dogs and rats exposed to 21 ppm for 6 hours but the dogs became nauseated, coughed up a small amount of mucous material, and had rapid respiration and salivation [Horn and Weir 1955]. . . . Human data: It has been reported that 50 ppm or more may be fatal in 30 minutes to 2 hours [Deichmann and Gerarde 1969].
Lethal Concentration
LC50 (rat) = 299 ppm/1H
Reference Link #2
NFPA
will not burn
ERPG-1
0.1 ppm
ERPG-2
1 ppm
ERPG-3
10 ppm
Adverse Effects
Toxic Pneumonitis
Yes
Dermatotoxin
Skin burns
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent: