Sodium amide
Agent Name
Sodium amide
CAS Number
7782-92-5
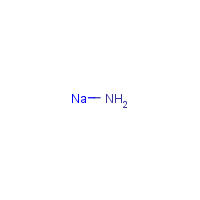
Formula
H2-N-Na
Major Category
Nitrogen Compounds
Synonyms
Sodamide; [ChemIDplus] UN1390
Category
Other Nitrogen Compounds
Description
Solid; Commercial form is white to olive-green; Quickly absorbs water and carbon dioxide from atmosphere; [Merck Index] White solid with an odor of ammonia; [Hawley] Grey powder or granules with an ammoniacal odor; [Alfa Aesar MSDS]
Sources/Uses
Used as a dehydrating agent, in ammonolysis reactions, in Claisen condensations, and for alkylation of nitriles and ketones; Also used to make indigo, hydrazine, ethynyl compounds, acetylenic carbinols, and sodium cyanide; [Merck Index]
Comments
Emergency treatment: "Corrosives- alkaline"; [HSDB] Reacts violently with water producing sodium hydroxide and ammonia; Oxidation products after air exposure may be highly explosive; A severe skin, eye, and mucous membrane irritant; [Merck Index] A strong reducing agent; An explosive peroxide may be produced on storage; May cause caustic burns to skin and eyes; [CAMEO] Reacts violently with water evolving highly flammable gases; Explosive on contact with oxidizing substances; A highly corrosive substance that can cause injury to the skin, eyes, and respiratory tract; Inhalation may cause chemical pneumonitis; [Alfa Aesar MSDS]
Biomedical References
Exposure Assessment
Explanatory Notes
The Guide in the Emergency Response Guidebook is for "Alkali metal amides."
NFPA
may ignite at ambient temp
Adverse Effects
Toxic Pneumonitis
Yes
Dermatotoxin
Skin burns
Diseases, Processes, and Activities Linked to This Agent
Other Information
No other related information on this agent was found.