1,1,1-Trichloroethane
Agent Name
1,1,1-Trichloroethane
Alternative Name
Methyl chloroform
CAS Number
71-55-6
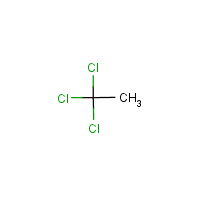
Formula
C2-H3-Cl3
Major Category
Solvents
Synonyms
TCA; Chloroethene; Methyl chloroform; Methyltrichloromethane; Trichloromethylmethane; alpha-Trichloroethane; alpha-T; [ATSDR CaseStudies # 24] Trichloroethane, 1,1,1-; 1,1,1-TCE; 1,1,1-Trichloraethan [German]; 1,1,1-Tricloroetano [Italian]; Aerothene TT; Baltana; CF 2; Chloroform, methyl-; Chlorotene; Chlorothene; Chlorothene NU; Chlorothene SM; Chlorothene VG; Chlorothene, inhibited; Chlorten; Cleanite; Dowclene LS; Ethana NU; Ethane, 1,1,1-trichloro-; F 140a; Genklene LB; HCC 140a; ICI-CF 2; Inhibisol; Methylchloroform; Solvent 111; Tafclean; Tcea; Three One A; Three One S; Trichloro-1,1,1-ethane [French]; Trichloroethane; [ChemIDplus] Aerothene; Chloroetene; Chloroethene NU; Chlorothane NU; Strobane; Tri-ethane; 1,1,1-Trichlorethane; [CAMEO] TCEN; [NTP] UN2831
Category
Chlorinated Aliphatics
Description
Colorless liquid with a mild, chloroform-like odor; [NIOSH] Clear colorless liquid with a sweet odor; [Matheson Tri-Gas MSDS]
Sources/Uses
Used for degreasing, cleaning, and thinning; [LaDou, p. 552] Used in photography (film cleaner); [www.ci.tucson.az.us/arthazards/medium.html] Used as a solvent for natural and synthetic resins, oils, waxes, tar, alkaloids, adhesives, coatings, photoresist polymers, textile dyeing, and extractions, as dry cleaning agent, for cleaning metal, plastic molds, electrical equipment, motors, electronic components and instruments, missile hardware, paint masks, photographic film, printed circuit boards, and certain plastics components, in textile processing, metal cutting oils, inks, drain cleaners, aerosols, vapor degreasing, as chemical intermediate, and pesticide; [HSDB] Also used in the leather processing and paper industries; [IUCLID] No active pesticide product registrations in the US; [NPIRS]
Comments
For the general population, the most likely sources of exposure are home consumer products, building products, and contaminated food and water. TCA was used as a general anesthetic. Inhalation abuse of TCA can result in "sudden sniffing death," from cardiac dysrhythmias. [ATSDR Case Studies #24] Cardiac sensitization and low hepatotoxic potential have been documented in animal studies. [ACGIH] 1,1,1-Trichloroethane causes "trivial hepatotoxicity, unless exposure is very heavy or agent ingested." [Zimmerman, p. 333] Methyl chloroform is in the list of "Some volatile substances which may be abused by inhalation" published on the web site of the U.N. International Drug Control Programme, indicating its potential to cause narcosis in workers. [Flanagan et al. Volatile Substance Abuse] A mild skin, eye, and respiratory tract irritant; Inhalation of high concentrations can cause CNS depression and cardiac arrhythmias; [ICSC] May cause smarting and reddening of skin if spilled and allowed to remain on clothes; High vapor concentrations may cause mild smarting of eyes and respiratory system; Inhalation may cause loss of equilibrium, incoordination, and unconsciousness; [CHRIS] An irritant and CNS depressant; May cause liver and kidney injury; [Matheson Tri-Gas MSDS]
Restricted
"Because 1,1,1-trichloroethane damages the ozone layer, production in the United States was phased out in 1996, but supplies as a raw material will be available until the year 2002." [ATSDR Medical Management]
Reference Link #1
Biomedical References
Exposure Assessment
BEI
Methyl chloroform in end-exhaled air = 40 ppm prior to last shift of workweek; for trichloroethanol in blood and urine and TCA in urine BEIs, see the ACGIH booklet;
Skin Designation (ACGIH)
Insufficient data
TLV (ACGIH)
350 ppm
STEL (ACGIH)
450 ppm
PEL (OSHA)
350 ppm
MAK
100 ppm
IDLH (NIOSH)
700 ppm
Excerpts from Documentation for IDLHs
Other animal data: No significant signs of intoxication were seen in rats inhaling 500 ppm, 6 hours per day for 4 days [Savolainen et al. 1977]; in mice inhaling up to 1,300 ppm for 1 hour [Kjellstrand et al. 1985]; in rats inhaling up to 3,000 ppm for 0.5 to 4 hours [Mullin and Krivanek 1982]; or in baboons inhaling up to 1,400 ppm for 4 hours [Geller et al. 1982]. \
Human data: The onset of central anesthesia has occurred in individuals exposed for up to 7 hours to concentrations approaching 500 ppm [Stewart et al. 1969]. It has been stated that exposure to 900 to 1,000 ppm causes prompt, though minimal impairment of coordination; obvious disturbances in equilibrium have been noted above 1,700 ppm [MCA 1965]. Those exposed to 800 to 1,000 ppm have exhibited early anesthetic effects including incoordination [Stewart et al. 1961]. Volunteers exposed to 920 ppm for 5 to 45 minutes showed a slight loss of coordination and equilibrium [Stewart et al. 1961].
Vapor Pressure
124 mm Hg
Odor Threshold Low
16 ppm
Odor Threshold High
714 ppm
Lethal Concentration
LC50 (rat) = 18,000 ppm/4hr
Explanatory Notes
Detection odor threshold from AIHA (mean = 390 ppm); VP from HSDB;
Half Life
Blood to expired air: 1-9 hours; trichloroethanol (blood): 10-27 hours; trichloroacetic acid (blood): 70-85 hours; [TDR, p. 1173]
Reference Link #2
NFPA
must be preheated
ERPG-1
350 ppm
ERPG-2
700 ppm
ERPG-3
3,500 ppm
Adverse Effects
Neurotoxin
Acute solvent syndrome
Hepatotoxin
Hepatoxic (a) from occupational exposure (secondary effect) or (b) in animal studies or in humans after ingestion
IARC Carcinogen
Probable (2a)
ACGIH Carcinogen
Not Classifiable
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent:
Processes
Industrial Processes with risk of exposure:
Activities
Activities with risk of exposure: