Sulfur dichloride
Agent Name
Sulfur dichloride
CAS Number
10545-99-0
Formula
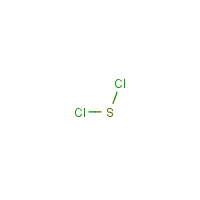
Cl2-S
Major Category
Other Classes
Synonyms
Chlorine sulfide; Dichlorosulfane; Disulfur tetrachloride; Monosulfur dichloride; Sulfur chloride; Sulphur dichloride; [ChemIDplus] UN1828
Category
Sulfur Compounds
Description
A fuming red to reddish-brown liquid with a chlorine odor; [HSDB]
Sources/Uses
Used as a chemical intermediate, a chlorinating agent, a vulcanizing agent, a sulfur solvent, and an additive to purify sugar juices; Also used in metallurgy; [HSDB]
Comments
Corrosive to skin; [Quick CPC] Releases HCL on contact with moisture and can cause irritation and burns to the upper respiratory tract and lungs; Humans tolerate 4.7 ppm without apparent adverse effects, but 30 ppm could be harmful if exposed daily for longer than one month. [HSDB] Reacts with water producing hydrogen chloride; Corrosive to skin, eyes, and respiratory tract; Inhalation may cause pulmonary edema; [ICSC] Sulfur chlorides release HCl, SO2, and H2S when spilled in water; [ERG 2016]
Biomedical References
Exposure Assessment
TIH
Yes
Dangerous When Wet
Yes
Odor Threshold Low
0.001 ppm
Explanatory Notes
It decomposes in water; boiling point = 59 degrees C; [HSDB] The Guide in the Emergency Response Guidebook is for "Sulfur chlorides." Some “Water Reactive Materials” are also TIH materials themselves: Sulfur chlorides; [ERG 2016]
NFPA
must be preheated
Adverse Effects
Toxic Pneumonitis
Yes
Dermatotoxin
Skin burns
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent:
Processes
Industrial Processes with risk of exposure: