Aluminum fluoride
Agent Name
Aluminum fluoride
CAS Number
7784-18-1
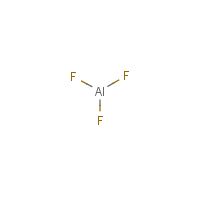
Formula
Al-F3
Major Category
Metals
Synonyms
Aluminium fluorure [French]; Aluminum trifluoride; Fluorid hlinity [Czech]; [ChemIDplus] UN1759
Category
Metals, Inorganic Compounds
Description
White solid; [Hawley] Hygroscopic; [ICSC] Odorless powder; [MSDSonline]
Sources/Uses
Used in ceramics; as flux in metallurgy, ceramic glazes, and enamels; in aluminum manufacture (to lower the melting point and increase the conductivity of the electrolyte); as inhibitor of fermentation; as catalyst in organic reactions; in the manufacture of aluminum silicate; [HSDB]
Comments
A severe eye irritant; A respiratory tract irritant; [HSDB] A skin, eye, and respiratory tract irritant; "Repeated or prolonged inhalation exposure may cause asthma. The substance may have effects on the bone, nervous system, resulting in bone alterations (fluorosis), and nervous system impairment." [ICSC] A corrosive substance that can cause injury to the skin, eyes, and respiratory tract; Inhalation may cause chemical pneumonitis; [MSDSonline] See "FLUORIDES."
Biomedical References
Exposure Assessment
BEI
Fluorides in urine = 2 mg/L prior to shift or 3 mg/L at end of shift; (Repeated measurements recommended.)
TLV (ACGIH)
2.5 mg/m3, as F
PEL (OSHA)
2.5 mg/m3, as F
MAK
1 mg/m3, inhalable fraction, as F
Explanatory Notes
VP = 1 mm Hg at 1238 deg C; [HSDB] The Guide in the Emergency Response Guidebook is for "Corrosive solid, n.o.s."
Adverse Effects
Asthma
Yes
Toxic Pneumonitis
Yes
Dermatotoxin
Skin burns
Diseases, Processes, and Activities Linked to This Agent
Diseases
Occupational diseases associated with exposure to this agent:
Processes
Industrial Processes with risk of exposure:
Activities
Activities with risk of exposure: