Oxalic acid, anhydrous
Agent Name
Oxalic acid, anhydrous
CAS Number
144-62-7
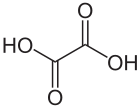
Formula
C2-H2-O4
Major Category
Other Classes
Synonyms
Acide oxalique [French]; Acido ossalico [Italian]; Acidum oxalicum; Aktisal; Aquisal; Ethanedioic acid; Kyselina stavelova [Czech]; Oxaalzuur [Dutch]; Oxalate; Oxalic acid; Oxalsaeure [German]; Oxiric acid; [ChemIDplus] Dicarboxylic acid; [ACGIH]
Category
Organic Acids
Description
Colorless, odorless powder or granular solid. [Note: The anhydrous form (COOH)2 is an odorless, white solid.]; [NIOSH]
Sources/Uses
Used in metal cleaning, chemical synthesis, dye and rubber manufacturing, and textile stripping/finishing; Also used as a disinfectant (swimming pool and drainage system) and bathroom sanitizer; [ACGIH] Several species of plants contain soluble and insoluble oxalate salts. [Olson, p. 360] Used in photography (toner and platinum printing); Also used as a mordant in textile dyeing; [www.ci.tucson.az.us/arthazards/medium.html]
Comments
Liquid causes second or third degree burns after short contact. [CHRIS] TLV Basis is irritation (eye, skin and upper respiratory tract); [ACGIH] Solutions range from irritating to corrosive. Inhalation of high concentrations may induce chemical pneumonitis. Ingestion of oxalic acid or soluble oxalate compounds causes acute hypocalcemia and deposition of calcium oxalate crystals in the brain, heart, kidney, and other organs. In plant poisoning cases, ingestion of insoluble oxalate compounds causes irritation and swelling of the mouth, throat, and esophagus. [Olson, p. 360-1] A corrosive substance that can cause pulmonary edema after inhalation of aerosol; [ICSC] May cause kidney damage, hypocalcemia, and hepatic necrosis in severe poisoning cases following ingestion; [HSDB]
Reference Link #1
Biomedical References
Exposure Assessment
Skin Designation (ACGIH)
Insufficient data
TLV (ACGIH)
1 mg/m3
STEL (ACGIH)
2 mg/m3
PEL (OSHA)
1 mg/m3
IDLH (NIOSH)
500 mg/m3
Excerpts from Documentation for IDLHs
Human data: It has been reported that the lethal oral dose is 15 to 30 grams [Webster 1930]. [Note: An oral dose of 15 to 30 grams is equivalent to a 30minute exposure to 10,000 to 20,000 mg/m3 assuming a 50 liter per minute breathing rate and 100% absorption.]
Vapor Pressure
0.000234 mm Hg
Explanatory Notes
VP = 0.54 mm Hg @ 105 deg C; [HSDB]
NFPA
must be preheated
Adverse Effects
Toxic Pneumonitis
Yes
Hepatotoxin
Hepatoxic (a) from occupational exposure (secondary effect) or (b) in animal studies or in humans after ingestion
Dermatotoxin
Skin burns
Diseases, Processes, and Activities Linked to This Agent
Processes
Industrial Processes with risk of exposure:
Activities
Activities with risk of exposure: